Engineering Services
FDNA Engineers and Equipment Supply, with support of its technical staff and strong engineering Background, has the ability to perform consulting activities, basic design and detailed design, preparation of technical specifications for equipment. In general, the scope of activity of this company is related to the manufacture of equipment in the oil, gas, petrochemical and energy industries.
Basic Design and PDP:

Basic Engineering Design
FDAN provides Basic Engineering Package services for each client. This package typically includes the following deliverables:
We can make complete basic Design and PDP for different process.
Scope of Work:

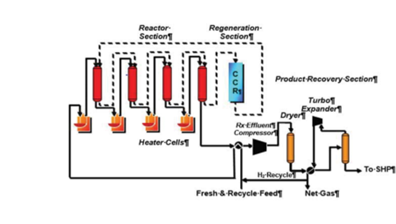
BENEFITS AND ADVANTAGES OF UOP OLEFLEX TECHNOLOGY.
Since its commercialization in 1990, numerous technology advancements to the
Oleflex Process Unit have resulted in lower production costs, increased on-stream
time, and larger economies of scale. The Oleflex Process offers an economically
attractive and reliable source of propylene and optionally, hydrogen, with additional
benefits and advantages discussed below:
Most Effective Use of Capital
Lowest Operating Cost
Minimal Coke Formation
Catalyst Performance
Most Reliable Technology
Environmentally Sound Catalyst System
Lowest Environmental Footprint and Ease of Permitting
The PDH is a catalytic technology (CATOFIN: fixed-bed type of ABB Lummus and OLEFLEX: moving-bed type of UOP) utilised for the conversion of propane intopropylene, and various catalysts have been developed to increase the propylene yield over recent decades (monometallic Pt nanoparticles).

In each reactor, a pump circulates the reaction slurry mixture, containing 55 percent solids, at high velocity to avoid solids settlement and improve heat transfer. Cooling water is circulated through the jacket for heat removal. The total reactor residence time is 1.4 hours for impact copolymer production. For homopolymer production, the residence time is about one hour. Product transition time may vary from one to two hours (maximum) for homopolymer transitions, to a maximum of six hours for the worst-case transition between homopolymer and butene-1 copolymer families.
Part of the circulating mixture is withdrawn from the reactor and flashed through a heated, jacketed pipe into a high-pressure degasser, which is operated at about 18 barg (260 psig), where the powder separates from the monomer. The monomer vapor leaving the flash tank is condensed against cooling water and is pumped back to the reactor.
The polymer powder from the degasser, still containing some monomer, flows to a low-pressure flash filter or to a copolymer reactor if high impact copolymer is being produced. The low- pressure filter operates at 0.7 barg (10 psig). The recovered vapors are compressed to 18 barg (260 psig) and are combined with the high-pressure monomer vapor for condensation and recycle to the reactor. For the production of impact copolymer, ethylene is separated and recycled.
In high impact copolymer production, the powder from the high-pressure degasser (with active polymerization catalyst) enters a gas phase reactor where ethylene and additional propylene are added. Cooling is provided by means of a gas recirculation loop. This reactor is operated at 10 to 14 barg (140 to 200 psig) and at 70 to 80°C (158 to 176°F) and is constructed of carbon steel. The proposed plant operates two fluid-bed, gas phase copolymer reactors in series for the production of low blush copolymer (not shown). Levels of up to 35 to 36 weight percent of rubber phase in the polymer are commercially achieved by using a single-stage copolymer reactor system, even if a two-gas phase reactor configuration can provide a better production rate for specialty impact copolymer products.
The polymer from the low-pressure filter (with a monomer content of 1,000 to 2,000 ppm) is sent to a monomer stripping system for the removal of residual monomer. Here, steam strips all hydrocarbons, which are recovered, dried, and recycled back to the low-pressure degassing step or made available to the battery limits for credit for propane purge when using polymer grade propylene. Wet polymer from the steamer is dried by a hot nitrogen closed loop. The residual monomer content in the polymer leaving the steaming and drying section is 3 to 5 ppm.
Process Description Polypropylene Plant
The proposed bulk process uses a loop reactor design is shown in Figure 1.1 and Figure 1.2. The plant consists of a catalyst feed system, a reactor system followed by monomer flash, recycle and stripping, and extrusion and compounding.
The process can operate with a single loop reactor to produce the full range of products, but plants with medium/large capacities are generally designed with two loop reactors in series. This gives the capability of producing bimodal resins to increase mechanical properties (e.g., for BOPP). Therefore, this design includes two loop reactors in series followed by the impact copolymer reactor (gas phase).
Monomer feed treatment normally includes guards to remove catalyst poisons (e.g. oxygen and sulfur containing compounds, etc.). Chemical grade propylene can be used directly in polymerization without preliminary propane removal. Propylene can be recovered directly from a liquid fraction of unreacted propylene/propane mixture purged from the plant to upstream facilities (such as the splitting unit).
In the process, fresh propylene is combined with recycle propylene from the flash step and fed to each reactor. A small pre-reactor (loop) is employed to initiate the polymerization (not shown). The catalyst
Hydrogen is also fed to the reactors to control molecular weight. The homopolymer reaction area consists of two loop reactors in series, each consisting of legs (4 to 6) depending on the capacity. The reactors are constructed of calmed (low temperature) carbon steel and operate at 75 to 80°C (167 to 176°F) and 40 to 45 barg (580 to 650 psig). This is a higher and wider operating window than in the past, allowing for better catalyst performance, giving, for example, polymers with higher crystallinity combined with high isotacticity. Catalyst activities are typically 40 thousand to 50 thousand tons of polymer produced per ton of catalyst. Activities of up to 100 thousand tons per ton have been achieved for some experimental grades/catalysts trials at semi-commercial scale.


Acrylonitrile is a chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics.
Ammoxidation Reaction :
CH2=CHCH3 + NH3 + 1.5O2 CH2=CHCN + 3H2O
CH2=CHCH3 + 3NH3 + 3O2 3HCN + 6H2O
silica supported oxides of selenium, iron, tellurium
OSe1Fe0.83OTeO0.68
Conversion of 1st reaction is 90%
Conversion of 2nd reaction is 2 %
Convert mole fraction to mass fraction
Mole fraction(x) = mole of I / total mole
Mass fraction (w) = mass of I / total mass
X*Mw = mass of i / total mole ______(1)
* Mw = total mass / total mole _____(2)
(1) / (2) = mass fraction
Acrylonitrile Butadiene Styrene (ABS)

ABS is an ideal material wherever superlative surface quality, colorfastness and luster are required. ABS is an extremely cost-effective material for components with stringent service requirements, or where there is weight-saving potential.
ABS standard grades have been developed specifically to meet the requirements of major customers. ABS is readily modified both by the addition of additives and by variation of the ratio of the three monomers Acrylonitrile, Butadiene and Styrene: hence grades available include high and medium impact, high heat resistance, and electroplatable. Fibre reinforcement can be incorporated to increase stiffness and dimensional stability. ABS is readily blended or alloyed with other polymers further increasing the range of properties available. Fire retardancy may be obtained either by the inclusion of fire retardant additives or by blending with PVC. The natural material is an opaque ivory color and is readily colored with pigments or dyes. Transparent grades are also available.
A variety of grades are available for different applications, the material is typically injection molded or extruded.

Acrylonitrile Butadiene Styrene (ABS) and Other Specialist Styrenics
Acrylonitrile Butadiene Styrene (ABS) is an ideal material wherever superlative surface quality, colorfastness and luster are required. ABS is a two phase polymer blend. A continuous phase of styrene-acrylonitrile copolymer (SAN) gives the materials rigidity, hardness and heat resistance. The toughness of ABS is the result of sub microscopically fine polybutadiene rubber particles uniformly distributed in the SAN matrix.
History
Styrene Acrylonitrile copolymers have been available since the 1940’s and while its increased toughness over styrene made it suitable for many applications, its limitations led to the introduction of a rubber (butadiene) as a third monomer and hence was born the range of materials popularly referred to as ABS plastics. These became available in the 1950’s and the variability of these copolymers and ease of processing has led to ABS becoming the most popular of the engineering polymers.

Typical Usage
Automotive
Automotive construction places particularly high requirements on the materials used. Under extreme stresses they have to be dimensionally stable and must not warp, even when faced with great temperature variations.
Electrical and Electronic (E&E)
Electrical and electronics industries increasingly require surfaces which are not only highly scratch- and wear-resistant but also decorative and easy to maintain. ABS’s excellent antistatic performance is a particular advantage here.
Office Equipment
Attractive products with elegant design and high quality create a feeling of well-being at home and in the office. ABS can create exciting and varied color schemes. Grades can also be electroplated, emboss-stamped or metallized.
Depending on the molding equipment used. Surfaces can be matt, glossy or satin
Applications
Because of its good balance of properties, toughness/strength/temperature resistance coupled with its ease of molding and high quality surface finish, ABS has a very wide range of applications. These include domestic appliances, telephone handsets computer and other office equipment housings, lawn mower covers, safety helmets, luggage shells, pipes and fittings. Because of the ability to tailor grades to the property requirements of the application and the availability of electroplatable grades ABS is often found as automotive interior and exterior trim components
EPDM Rubber

Ethylene-Propylene-(Diene) rubber EPDM
EPDM is one of the most worldwide used polymers, employed in a large variety of applications. Versalis EP(D)M is produced by suspension copolymerization, without the use of solvent, initiated by metalorganic component (Ziegler-Natta catalysts). In this work, a first-principle model to evaluate the performance of an industrial EP(D)M plant production is proposed. The polymerization mechanism was implemented in an Aspen Polymers v12.1 simulation of the industrial plant. Model parameters were tuned based on process data for selected product grades; model performance was cross-validated with further product grades and production line data. By leveraging external code (e.g., MATLAB/Python), process engineers can automate the tuning procedure, and accelerate sensitivity/optimization analysis. Both steady-state and dynamic simulations can successfully be employed to improve process understanding, analyze past conditions, monitor plant performance, and explore future process/product developments.
Ethylene-propylene elastomers are synthetic rubbers that are prepared by polymerization of ethylene, propylene, and optionally a non-conjugated diene. They are random, amorphous polymers with outstanding resistance to ozone, ageing, weather, and high temperatures due mainly to their saturated backbone structure. Main applications for ethylene-propylene elastomers include automotive parts, single-ply roofing, appliance parts, modification of other polymers (thermoplastic olefins), wire and cable, hoses, and viscosity index improvers for lubricating oils. Two types of ethylene-propylene elastomers are currently produced: (i) Ethylene Propylene Copolymers, which contain a saturated chain and require vulcanization by means of free radical generators such as organic peroxides or a combination of peroxides and Sulphur, and (ii) Ethylene-Propylene Terpolymers, which are essentially ethylene-propylene-diene terpolymers that have a saturated chain and a diene in the side chain.


The first EP(D)M production plant via suspension technology (60 kt/y) is on stream since 1974 at Ferrara (Italy); in 1991 a new line was built increasing the plant capacity up to 100 kt/y, and in 2018 a further line with a capacity of 48 kt/y was started-up. Two more lines, with an overall capacity of 96 kt/y, are on stream since 2017 in the Far East. In the following sections, EP(D)M polymerization and industrial plant are briefly introduced.
PET / PTA

Polyethylene terephthalate PET
The plastic polyethylene terephthalate, or PET for short, celebrated its 80th birthday in 2021. Originally developed as an alternative for the production of textile fibres, the material is now one of the most important in the packaging and textile industries. PET has become known around the world through its use in the production of beverage bottles.
The first PET bottles appeared on the market on a large scale in the USA in the early 1970s. Soft drink manufacturers in particular have used the material to bottle their products, heralding the triumphant advance of the recyclable material. In contrast to conventional glass bottles, PET offers break resistance, lower weight and easier handling.
Purified Terephthalic Acid (PTA)
Purified Terephthalic Acid (PTA) is a type of aromatic dicarboxylic acid. It is a white powdered crystal under normal atmospheric temperature being characteristic of non-toxicity and inflammability. PTA is produced in two main process steps. The 1st step is an oxidation process of paraxylene as main raw material and using acetic acid as solvent. The product is called Crude Terephthalic Acid (CTA) as it significant amount of impurities. The 2nd step is is hydrogenation process of CTA to remove the impurities. The final product is called PTA. Polyester fibers are used mainly in rugs, clothing, furniture and industrial applications, as well as other consumer products.
Application :
- Fiber & Filament Yarn
- PET Bottle
- PET Film and Others
